The Chinese government is actively creating an open and fair market environment for the development of China's pharmaceutical industry, and vigorously promoting its sustained, rapid and healthy development. Through its unremitting efforts in the past 60 years or so since the founding of the People's Republic of China in 1949, especially in the past 30 years since the adoption of the reform and opening-up policies in 1978, China has not only reversed the situation of inadequacy of medical services and drugs, but also remarkably elevated its guarantee capability regarding the quality and safety of drugs.
At present, China can produce 1,500 types of drug substances, many of which lead the world in terms of output, including penicillin and vitamin C. A number of botanic and natural drugs, such as anti-infective berberine and anti-tumor colchicine have been mass-produced and widely used in China. China's antibiotic, vitamin, hormone, antipyretic and analgesic, amino acid, and alkaloid products take up considerable shares of the international pharmaceutical market. China's artemisinin products are used all over the world, significantly contributing to the international anti-malaria efforts. Today, China can produce over one billion doses a year of 41 types of vaccines against infection caused by 26 kinds of viruses and pathogenic bacteria. Among them, the country's annual output of vaccines for preventing common infectious diseases such as hepatitis B, poliomyelitis (infantile paralysis), measles, pertussis, diphtheria and tetanus, can serve 500 million people. Besides meeting the domestic demand, China also provides vaccines to the World Health Organization (WHO) for disease prevention in other countries. China produces more than 3,000 types of medical devices, among which high-tech diagnosis and treatment products such as the digital X-ray, magnetic resonance, ultrasonic and computed tomography apparatus hold considerable market shares. By the end of 2007, China had 12,591 enterprises producing medical devices, and 6,913 pharmaceutical enterprises (including producers of prepared slices of Chinese crude drugs and oxygen for medical use), of which 4,682 were producers of active pharmaceutical ingredients and preparations.
Figure 1 Growth of Total Output Value of China's Pharmaceutical Industry (composed of seven sub-industries, at current prices)
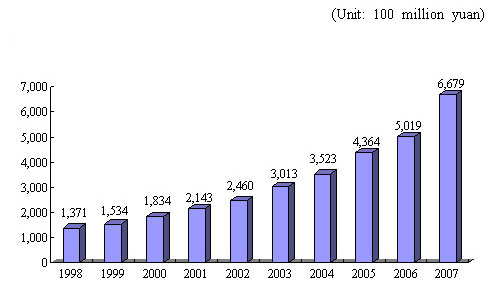
Recent years have witnessed a marked increase in the total output value and trade volume of China's pharmaceutical industry, which is divided into seven categories ?a Chinese patent medicines, prepared slices of Chinese crude drugs, bulk chemical drug substances, chemical drug preparations, biologicals, medical devices and hygienic materials. Their total output value rose from 137.1 billion yuan in 1998 to 667.9 billion yuan in 2007. From 1998 to 2007, the export trade volume of pharmaceutical industry increased from US$ 3.4 billion to US$ 24.6 billion, and the import trade volume from US$ 1.5 billion to US$ 14.0 billion.
To prevent generic drugs from freely using the research and development data of new drugs, and therefore hindering the motivation for inventing new drugs, China earnestly fulfills its commitments to the WTO and implements a data protection system for drugs. The Regulations for the Implementation of the Drug Administration Law of the People's Republic of China amended in 2002 stipulates that undisclosed data of drug studies and others which are independently acquired and submitted by drug manufacturers or sellers who eventually obtained production or marketing approval for the drugs in question which contain new chemical entities enjoy a six-year protection period.
Furthermore, the state implements special evaluation and approval procedures to encourage the invention of new drugs and the development and research of new drugs for treating difficult, complicated and severe diseases. The special procedures are applicable to the following new drugs: active ingredients which are extracted from plants, animals, minerals and other materials and have not been marketed in China, and preparations containing these ingredients; newly discovered medicinal materials and their preparations; drugs made of chemical materials which have not been marketed domestically or internationally and their preparations and biologicals; new drugs with obvious clinical advantages for treating AIDS, malignant tumors and rare diseases; and new drugs for diseases without effective treatment. From 1998 to the end of 2007, altogether 78 Class I new drugs obtained approval. The manufacturing techniques and levels of pharmaceutical enterprises greatly improved, and a series of new techniques and methods were invented, such as vitamin C two-step fermentation, berberine synthesis, and techniques and apparatus for manufacturing high-purity urokinase. New techniques for the fungal screening, breeding and fermentation of high-yield penicillin spores and the fermentation of cephalosporin C have reached the international advanced level. China can manufacture medium-sized medical equipment for export, and is moving toward the world's top rankings in respect of research into techniques for wearable device, bio-medical materials and tissue engineering.
The state has expedited the construction of modern pharmaceutical logistics and chain pharmacies, effectively guaranteeing public access to pharmaceutical product. By the end of 2007, China had 13,000 wholesale pharmaceutical enterprises, 341,000 retail pharmaceutical enterprises and chain store enterprises, and 554,000 rural drug supply outlets, fully satisfying the public's needs for drugs. As the Chinese people's standard of living continues to improve, the per-capita consumption level of drugs is gradually rising, reaching 332 yuan in 2006.
China has established a network for reporting and monitoring adverse drug reactions (ADR). In 1998 China officially joined the WHO Collaborating Center for International Drug Monitoring. In 2004 the state promulgated the Measures on Administration of Reporting and Monitoring of Adverse Drug Reactions, thereby formally adopting a system of reporting and monitoring ADR. By the end of 2002 ADR monitoring institutions at the provincial level and over 200 centers and stations below the provincial level for this purpose had been set up in 31 provinces, autonomous regions and municipalities directly under the central government. By then, a nationwide information network for monitoring ADR had emerged, making it possible for electronic reporting and online real-time reporting. Since 2000 China has made visible progress in ADR reporting. In 2007, some 400 cases of ADR per one million people were reported, a ratio approaching that of the developed countries. This demonstrated the considerable improvement in China's monitoring and early-warning capability regarding ADR. Drug administration departments promptly collect, evaluate and publish information about ADR. By the end of June, 2008, they had issued 13 bulletins in this respect, involving 44 types of drugs.
Meanwhile, drug administration departments have actively explored and enhanced drug re-evaluation work, and conducted pilot study, as well as retrospective study and surveys of the safety of drugs sold on the market. Through re-evaluation and study, the drug administration departments have revised the directions for puerarin, potassium dehydroandrograpolide succinate and andrographolide sodium bisulfite injections, abolished the pharmaceutical standard for manchurian aristolochia stem, revoked the manufacturing permission for bimolane, and suspended the selling and use of certain other types of drugs.
The state has reinforced its endeavors to establish the monitoring and re-evaluation system for the adverse event of medical devices. In 2004 this work was inaugurated nationwide. By the end of 2006, 31 provinces, autonomous regions and municipalities directly under the central government had set up provincial institutions to monitor the adverse event of medical devices, and an organizational framework for this purpose began to take shape. Based on the results of monitoring and re-evaluation, drug administration departments revoked the registration certificates for polyacrylamide hydrogel, ordered the recall of extracorporeal circulation circuits, and had dialysis powder re-registered.
Figure 2 Acceptance Rates of the 2007 Evaluation
Sampling of Drugs
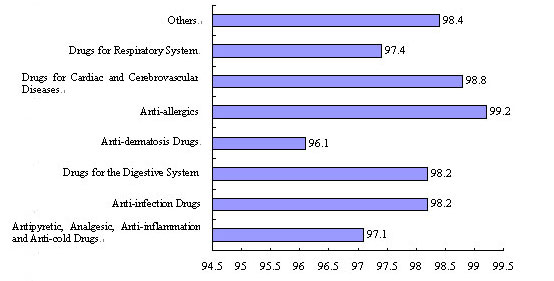
China has continuously intensified its supervision and post-market quality sampling of drugs to facilitate the steady improvement of drug quality and safety. In 2007 the state made an evaluation sampling program for 13,595 batches of TCM preparations, chemical drugs and biologicals, with an overall acceptance rate of 98 percent. Of the sampling products, there were 7,398 batches of chemical drugs, 2,586 batches of antibiotics and 3,611 batches of Chinese patent medicines, with acceptance rates of 98 percent, 98.1 percent and 97.6 percent, respectively. The influenza vaccine sampled had shown 100-percent acceptance rates for two consecutive years. Drug administration departments ordered the recall or withdrawal from the market of unqualified drugs and medical devices, exercised administrative control over them, and disposed of them according to law. At the same time, the state also adopted a series of measures to crack down on the manufacturing of counterfeit and inferior drugs so as to ensure safe use of drugs by the people.